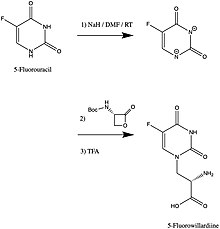
5-Fluorowillardiine
[4] It is an excitotoxic neurotoxin when used in vivo and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors in vitro.
[10] In another study, (S)-5-Fluorowillardiine showed biphasic dose-dependent neurotoxicity in cultural rodent cortical neurons, with EC50 values of 0.70 and 170 μM.
[11] While in vivo research is sparse, a study in 5-day-old mice injected with the closely related AMPA/kainate agonist (S)-5-Bromowillardiine showed cortical and white matter damage.
5-Fluorowillardiine exists as two distinct isomers: The particularly high affinity of 5-fluorowillardiine for the AMPA receptor is attributed to its fluorine substituent at the 5-position of the ring, which is electron-withdrawing and small enough to not interfere with binding.
Because the pKa values of halogenated willardiine derivates are approximately 8 (7.98 for 5-Fluorowillardiine), binding is mostly driven by an increase in entropy at physiological pH.