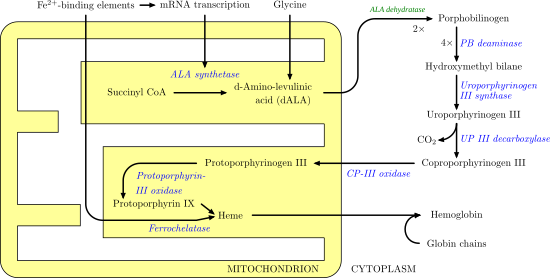
Aminolevulinic acid synthase
In humans, transcription of ALA synthase is tightly controlled by the presence of Fe2+-binding elements, to prevent accumulation of porphyrin intermediates in the absence of iron.
Gain of function mutations in the erythroid specific ALA synthase gene have been shown recently to cause a previously unknown form of porphyria known as X-linked-dominant protoporphyria.
[1] ALAS is a homodimer with similarly sized sub units and the active sites consisting of amino acid side chains such as arginine, threonine, and lysine exist at a subunity interface.
Although these three amino acids have been identified to allow this reaction to proceed, they would be inactive without the addition of cofactor pyridoxal 5’-phosphate (PLP) whose role in this synthesis is detailed in the image below.
Before the reaction can begin, the PLP cofactor binds to the lysine side chain to form a Schiff base that promotes attack by glycine substrate.
[1][8] In the detailed reaction mechanism, the hydronium atoms that are added in come from a variety of residues that offer hydrogen bonds to facilitate ALA synthesis.
This reaction mechanism is particularly unique relative to other enzymes that use the PLP cofactor because Glycine is initially deprotonated by a highly conserved active site lysine, leading to condensation with succinyl-CoA and loss of CoA.