Protein kinase B
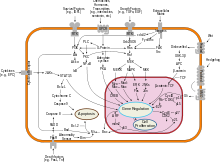
Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.
A mouse model with complete deletion of the Akt1 gene manifests growth retardation and increased spontaneous apoptosis in tissues such as testes and thymus.
[3] Since it can block apoptosis and thereby promote cell survival, Akt1 has been implicated as a major factor in many types of cancer.
In a mouse which is null for Akt1 but normal for Akt2, glucose homeostasis is unperturbed, but the animals are smaller, consistent with a role for Akt1 in growth.
[8] Akt isoforms are overexpressed in a variety of human tumors, and, at the genomic level, are amplified in gastric adenocarcinomas (Akt1), ovarian (Akt2), pancreatic (Akt2) and breast (Akt2) cancers.
The "Ak" in Akt refers to the AKR mouse strain that develops spontaneous thymic lymphomas.
The "t" stands for 'thymoma'; the letter was added when a transforming retrovirus was isolated from the Ak mouse strain, which was termed "Akt-8".
[15] Studies have suggested that cAMP-elevating agents could also activate Akt through protein kinase A (PKA) in the presence of insulin.
The phosphatases in the PHLPP family, PHLPP1 and PHLPP2 have been shown to directly de-phosphorylate, and therefore inactivate, distinct Akt isoforms.
[22] Akt1 can also activate NF-κB via regulating IκB kinase (IKK), thus result in transcription of pro-survival genes.
Moreover, activated Akt1 may enable proliferation and survival of cells that have sustained a potentially mutagenic impact and, therefore, may contribute to acquisition of mutations in other genes.
[citation needed] Akt1 regulates TFEB, a master controller of lysosomal biogenesis,[26] by direct phosphorylation at serine 467.
[27] Pharmacological inhibition of Akt promotes nuclear translocation of TFEB, lysosomal biogenesis and autophagy.
[30] Therefore, understanding the Akt proteins and their pathways is important for the creation of better therapies to treat cancer and tumor cells.
Treating the cells with Akt inhibitors before virus exposure leads to a significantly lower rate of infection.
In-frame duplications in the pleckstrin-homology domain (PHD) of the protein were found in more than 60% of JGCTs occurring in girls under 15 years of age.
The mutated proteins carrying the duplications displayed a non-wild-type subcellular distribution, with a marked enrichment at the plasma membrane.
This led to a striking degree of Akt1 activation demonstrated by a strong phosphorylation level and corroborated by reporter assays.
[41] Analysis by RNA-Seq pinpointed a series of differentially expressed genes, involved in cytokine and hormone signaling and cell division-related processes.
Further analyses pointed to a possible dedifferentiation process and suggested that most of the transcriptomic dysregulations might be mediated by a limited set of transcription factors perturbed by Akt1 activation.