AP-1 transcription factor
[2] The structure of AP-1 is a heterodimer composed of proteins belonging to the c-Fos, c-Jun, ATF and JDP families.
AP-1 was first discovered as a TPA-activated transcription factor that bound to a cis-regulatory element of the human metallothionein IIa (hMTIIa) promoter and SV40.
Fos was first isolated as the cellular homologue of two viral v-fos oncogenes, both of which induce osteosarcoma in mice and rats.
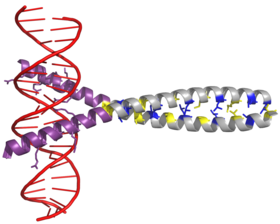
AP-1 transcription factor is assembled through the dimerization of a characteristic bZIP domain (basic region leucine zipper) in the Fos and Jun subunits.
Due to the amino acid sequence and the periodicity of the helices, the leucine side chains are arranged along one face of the α helix and form a hydrophobic surface that modulates dimerization.
AP-1 transcription factor has been shown to have a hand in a wide range of cellular processes, including cell growth, differentiation, and apoptosis.
AP-1 transcription factors are also associated with numerous physiological functions especially in determination of organisms’ life span and tissue regeneration.
AP-1 activity is induced by numerous extracellular matrix and genotoxic agents, suggesting involvement in programmed cell death.
[2] Increases in the levels of Jun and Fos proteins and JNK activity have been reported in scenarios in which cells undergo apoptosis.
[2] The AP-1 complex binds to a palindromic DNA motif (5’-TGA G/C TCA-3’) to regulate gene expression, but specificity is dependent on the dimer composition of the bZIP subunit.
Therefore, activity of AP-1 subunits in response to extracellular signals may be modified under conditions where the balance of keratinocyte proliferation and differentiation has to be rapidly and temporally altered.