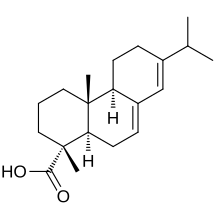
Abietic acid
Chemically, it is a complicated molecule featuring two alkene groups and a carboxylic acid within a chiral tricyclic framework.
Through the process of diagenesis, abietic acid changes into a collection of simpler compounds called abietanes.
In air and in the presence of certain cytochrome P450 enzymes, abietadiene is oxidized to abietic acid.
The stereochemistry of the typical abietane skeleton suggests a GGPP precursor with its fused cyclohexyl rings in a chair-chair ("normal") conformation, although some abietanes with alternative stereochemistry may be cyclized from CCP isomers containing alternative combinations of boat and chair cyclohexane conformers.
Laboratory procedures illustrate the nature of the extraction, which is the basis of a substantial industry, formerly known as naval stores.