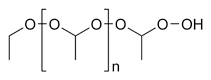
Ether
Oxygen is more electronegative than carbon, thus the alpha hydrogens of ethers are more acidic than those of simple hydrocarbons.
In the IUPAC Nomenclature system, ethers are named using the general formula "alkoxyalkane", for example CH3–CH2–O–CH3 is methoxyethane.
If the ether is part of a more-complex molecule, it is described as an alkoxy substituent, so –OCH3 would be considered a "methoxy-" group.
As for other organic compounds, very common ethers acquired names before rules for nomenclature were formalized.
Some toxins produced by dinoflagellates such as brevetoxin and ciguatoxin are extremely large and are known as cyclic or ladder polyethers.
Many classes of compounds with C–O–C linkages are not considered ethers: Esters (R–C(=O)–O–R′), hemiacetals (R–CH(–OH)–O–R′), carboxylic acid anhydrides (RC(=O)–O–C(=O)R′).
There are compounds which, instead of C in the C−O−C linkage, contain heavier group 14 chemical elements (e.g., Si, Ge, Sn, Pb).
Some ethers undergo rapid cleavage with boron tribromide (even aluminium chloride is used in some cases) to give the alkyl bromide.
[5] Depending on the substituents, some ethers can be cleaved with a variety of reagents, e.g. strong base.
Despite these difficulties the chemical paper pulping processes are based on cleavage of ether bonds in the lignin.
In addition to avoiding storage conditions likely to form peroxides, it is recommended, when an ether is used as a solvent, not to distill it to dryness, as any peroxides that may have formed, being less volatile than the original ether, will become concentrated in the last few drops of liquid.
The presence of peroxide in old samples of ethers may be detected by shaking them with freshly prepared solution of a ferrous sulfate followed by addition of KSCN.
This reactivity is similar to the tendency of ethers with alpha hydrogen atoms to form peroxides.
The dehydration of alcohols affords ethers:[7] This direct nucleophilic substitution reaction requires elevated temperatures (about 125 °C).
Commercially important ethers prepared in this way are derived from isobutene or isoamylene, which protonate to give relatively stable carbocations.
Nucleophilic displacement of alkyl halides by alkoxides This reaction, the Williamson ether synthesis, involves treatment of a parent alcohol with a strong base to form the alkoxide, followed by addition of an appropriate aliphatic compound bearing a suitable leaving group (R–X).
Although popular in textbooks, the method is usually impractical on scale because it cogenerates significant waste.
Secondary and tertiary halides are prone to undergo E2 elimination on exposure to the basic alkoxide anion used in the reaction due to steric hindrance from the large alkyl groups.
Since phenols are acidic, they readily react with a strong base like sodium hydroxide to form phenoxide ions.