Acetoacetate decarboxylase
Acetoacetate decarboxylase (AAD or ADC) is an enzyme (EC 4.1.1.4) involved in both the ketone body production pathway in humans and other mammals, and solventogenesis in bacteria.
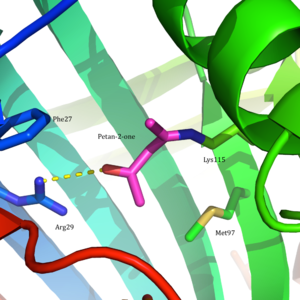
Specifically, the pKa value of lysine 115 in the active site is unusually low, allowing for the formation of a Schiff base intermediate and catalysis.
Acetoacetate decarboxylase is an enzyme with major historical implications, specifically in World War I and in establishing the state of Israel.
[3] During the war the Allies needed pure acetone as a solvent for nitrocellulose, a highly flammable compound that is the main component in gunpowder.
[4] In 1916, biochemist and future first president of Israel Chaim Weizmann was the first to isolate Clostridium acetobutylicum, a Gram-positive, anaerobic bacteria in which acetoacetate decarboxylase is found.
[3] This led the American and British governments to install the process devised by Chaim Weizmann in several large plants in England, France, Canada, and the United States.
The production of acetone by acetoacetate decarboxylase-containing or clostridial bacteria was utilized in large-scale industrial syntheses in the first half of the twentieth century.
[6] However, there has been a growing interest in acetone production that is more environmentally friendly, causing a resurgence in utilizing acetoacetate decarboxylase-containing bacteria.
The overall hydrophobic environment of the active site plays a critical role in favoring the neutral amine form of Lys115, a key residue involved in the formation of a Schiff base intermediate.
The first line of support for this mechanism came from a radiolabeling experiment in which researchers labeled the carbonyl group of acetoacetate with 18O and observed that oxygen exchange to water, used as the solvent, is a necessary part of decarboxylation step.
[9] These results provided support that the mechanism proceeds through a Schiff base intermediate between the ketoacid and an amino acid residue on the enzyme.
[2] If the pKa were not perturbed downward, the lysine residue would remain protonated as an ammonium cation, making it unreactive for the nucleophilic addition necessary to form the Schiff base.
Reaction of 5-NSA with acetoacetate decarboxylase and subsequent reduction of the resulting Schiff base with sodium borohydride led to the incorporation of a 2-hydroxy-5-nitrobenzylamino reporter molecule in the active site (Figure 2).
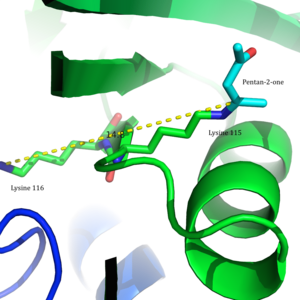
[12] These results were the basis for the proposal that the perturbation in the pKa of Lys115 was due to its proximity to the positively charged ε-ammonium group of Lys116 in the active site.
[8] In 2009, a crystal structure of acetoacetate decarboxylase from Clostridium acetobutylicum was solved, allowing Westheimer et al.'s proposal to be evaluated from a new perspective .
Acetic anhydride performs an electrophilic attack on the critical catalytic residue, Lys115, of acetoacetate decarboxylase to inactivate the enzyme.