Acetyl-CoA carboxylase
[1] The activity of ACC can be controlled at the transcriptional level as well as by small molecule modulators and covalent modification.
Biotin is covalently attached through an amide bond to the long side chain of a lysine reside in BB.
As BB is between BC and CT regions, biotin can easily translocate to both of the active sites where it is required.
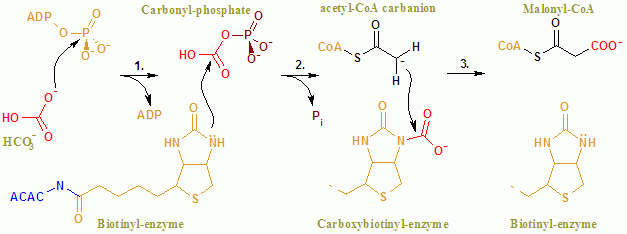
[8] The first reaction is carried out by BC and involves the ATP-dependent carboxylation of biotin with bicarbonate serving as the source of CO2.
The carboxyl group is transferred from biotin to acetyl-CoA to form malonyl-CoA in the second reaction, which is catalyzed by CT.
In the active site, the reaction proceeds with extensive interaction of the residues Glu296 and positively charged Arg338 and Arg292 with the substrates.
The PO43− deprotonates biotin, creating an enolate, stabilized by Arg338, that subsequently attacks CO2 resulting in the production of carboxybiotin.
[12][13] The regulation of mammalian ACC is complex, in order to control two distinct pools of malonyl-CoA that direct either the inhibition of beta oxidation or the activation of lipid biosynthesis.
[14] Mammalian ACC1 and ACC2 are regulated transcriptionally by multiple promoters which mediate ACC abundance in response to the cells nutritional status.
Activation of gene expression through different promoters results in alternative splicing; however, the physiological significance of specific ACC isozymes remains unclear.
[20] Phosphorylation can result when the hormones glucagon[21] or epinephrine[22] bind to cell surface receptors, but the main cause of phosphorylation is due to a rise in AMP levels when the energy status of the cell is low, leading to the activation of the AMP-activated protein kinase (AMPK).
[26] At the juncture of lipid synthesis and oxidation pathways, ACC presents many clinical possibilities for the production of novel antibiotics and the development of new therapies for diabetes, obesity, and other manifestations of metabolic syndrome.
[29] Firsocostat is under development in 2019 (Phase II)[30] by the pharmaceutical company Gilead as part of a combination treatment for non-alcoholic steatohepatitis (NASH), believed to be an increasing cause of liver failure.
[34] The heterogeneous clinical phenotypes of the metabolic disease combined malonic and methylmalonic aciduria (CMAMMA) due to ACSF3 deficiency are thought to result from partial compensation of a mitochondrial isoform of ACC1 (mACC1) for deficient ACSF3 in mitochondrial fatty acid synthesis (mtFASII).