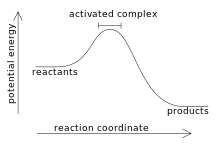
Activated complex
In chemistry, an activated complex represents a collection of intermediate structures in a chemical reaction when bonds are breaking and forming.
The activated complex is an arrangement of atoms in an arbitrary region near the saddle point of a potential energy surface.
[1] The region represents not one defined state, but a range of unstable configurations that a collection of atoms pass through between the reactants and products of a reaction.
Activated complexes have partial reactant and product character, which can significantly impact their behaviour in chemical reactions.
The theory is based on the idea that there is an equilibrium between the activated complex and reactant molecules.
[10] Error can arise from introducing symmetry numbers into the rotational partition functions for the reactants and activated complexes.
To reduce errors, symmetry numbers can by omitted by multiplying the rate expression by a statistical factor:
[10] The activated complex is a collection of molecules that forms and then explodes along a particular internal normal coordinate.
Ordinary molecules have three translational degrees of freedom, and their properties are similar to activated complexes.
However, activated complexed have an extra degree of translation associated with their approach to the energy barrier, crossing it, and then dissociating.