Outer sphere electron transfer
[1] In contrast, for inner sphere electron transfer the participating redox sites undergoing ET become connected by a chemical bridge.
The main theory describing the rates of outer sphere electron transfer was developed by Rudolph A. Marcus in the 1950s, for which he was awarded the Nobel Prize in Chemistry in 1992.
[2] A major aspect of Marcus theory is the dependence of the electron transfer rate on the thermodynamic driving force (difference in the redox potentials of the electron-exchanging sites).
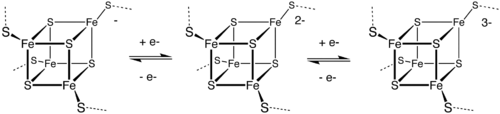
[3] Outer sphere electron transfer can occur between chemical species that are identical except for their oxidation state.
An example is the degenerate reaction between the tetrahedral ions permanganate and manganate: For octahedral metal complexes, the rate constant for self-exchange reactions correlates with changes in the population of the eg orbitals, the population of which most strongly affects the length of metal-ligand bonds: Outer sphere ET is the basis of the biological function of the iron-sulfur proteins.