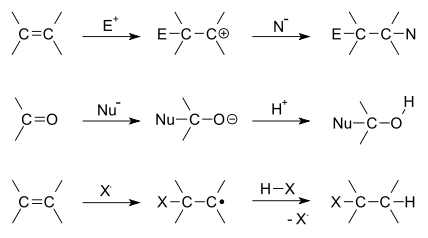
Addition reaction
[1][2] An addition reaction is limited to chemical compounds that have multiple bonds.
Another example is a compound that has rings (which are also considered points of unsaturation).
Depending on the product structure, it could promptly react further to eject a leaving group to give the addition–elimination reaction sequence.
Addition reactions are useful in analytic chemistry, as they can identify the existence and number of double bonds in a molecule.
For example, bromine addition will consume a bromine solution, resulting in a color change: Likewise hydrogen addition often proceeds on all double-bonds of a molecule, and thus gives a count of the number of a double and triple bonds through stoichiometry: