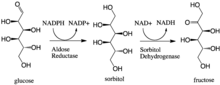Aldose reductase
The enzyme comprises 315 amino acid residues and folds into a β/α-barrel structural motif composed of eight parallel β strands.
[1] The reaction mechanism of aldose reductase in the direction of aldehyde reduction follows a sequential ordered path where NADPH binds, followed by the substrate.
[8] Kinetic studies have shown that reorientation of this loop to permit release of NADP+ appears to represent the rate-limiting step in the direction of aldehyde reduction.
[5][12][13] Thus, a [hydrogen-bonding] interaction between the phenolic hydroxyl group of Tyr-48 and the ammonium side chain of Lys-77 is thought to help to facilitate hydride transfer.
[5] Diabetes mellitus is recognized as a leading cause of new cases of blindness, and is associated with increased risk for painful neuropathy, heart disease and kidney failure.
Many theories have been advanced to explain mechanisms leading to diabetic complications, including stimulation of glucose metabolism by the polyol pathway.
Additionally, the enzyme is located in the eye (cornea, retina, lens), kidney, and the myelin sheath–tissues that are often involved in diabetic complications.
Additional reductase inhibitors such as Alrestatin, Exisulind, Imirestat, Zopolrestat, Tolrestat, Zenarestat, Caficrestat, Fidarestat, Govorestat, Ranirestat, Ponalrestat, Risarestat, Sorbinil, and Berberine, Poliumoside, Ganoderic acid[18] are currently in clinical trials.