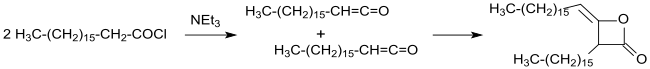Alkyl ketene dimer
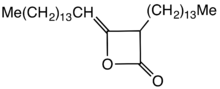
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene.
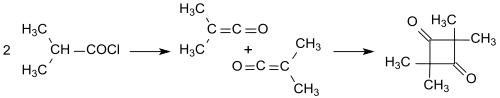
[7] [8] The primary reaction products of acid chlorides with hydrogen atoms in α-position and tertiary amines were identified by Hermann Staudinger[9][10] and Norman Thomas Mortimer Wilsmore[11] as highly reactive ketenes (ethenones) which form 2-oxetanones with an alkylidene group when dimerizing in a [2+2] photocycloadditions.
The industrial synthesis of alkylated ketenedimers (at that time still called ketoethenones) was patented in 1945 from long-chain carboxylic acid chlorides in inert solvents (such as diethyl ether or benzene) with triethylamine as tertiary amine under anhydrous conditions.
In addition to the significantly more reactive alkenylsuccinic anhydrides (which do also hydrolyze rapidly in the presence of water) alkylated ketene dimers have begun to be preferred surface and mass sizes in the paper industry from the 1960s onwards, beginning in the 1950s.
Aqueous alkyldiketene dispersions generally contain 10-20 wt% of AKD, as well as active protective colloids (particularly polycations such as cationic starch, copolymers of N-vinylpyrrolidone and quaternized N-vinylimidazole, acylated polyethyleneimines or cationic high molecular weight polyacrylamides with an average molar mass up to 7 million g/mol) and other stabilizers (usually anionic surfactants, for example ligninsulfonates or condensation products of naphthalenesulfonic acid sodium salt and formaldehyde).
[20] Such stabilized AKD dispersions are active and stable at room temperature for up to three months and also tolerate the addition of different fillers for paper or cardboard (e.g. kaolin, chalk, talc, titanium dioxide, calcium sulfate, aluminum oxide, etc.)
[21] Decisive criteria for the quality of the hydrophobicity of papers are The molecular structure (i.e. molar mass and cross-linking degree), the molar charge density of cationic groups, the exact dosage of the cationic polymer as a dispersion stabilizer and retention aid as well as keeping the other process parameters such as temperature, pH and residence times is crucial.
The hydrophobation of cellulose fibers with alkylated ketene dimers takes place most effectively in neutral or preferably weakly alkaline media (pH 7.5-9.0).
[21] The sizing with AKD is suitable for the permanent hydrophobation of newspaper, printing and writing paper and cardboard used as a container for liquids (including foodstuffs such as milk), as well as for the improvement of shape stability and runnability.