Alkylation unit
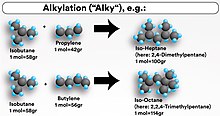
It is used to convert isobutane and low-molecular-weight alkenes (primarily a mixture of propene and butene) into alkylate, a high octane gasoline component.
In short, the alky produces a high-quality gasoline blending stock by combining two shorter hydrocarbon molecules into one longer chain gasoline-range molecule by mixing isobutane with a light olefin such as propylene or butylene from the refinery's fluid catalytic cracking unit (FCCU) in the presence of an acid catalyst.
The product of the unit, the alkylate, is composed of a mixture of high-octane, branched-chain paraffinic hydrocarbons (mostly isoheptane and isooctane).
In the gasoline summer pool, the content of alkylate can be as high as 15% because lower Reid vapor pressure (RVP) reduces the possibility to blend butane.
Indeed, in 1996 around 60% of the installed capacity was based on HF,[6] but since then this ratio has been reducing because during the last decade on 10 new alkylation units commissioned, more than 8 of them were SAAU.
Constructing a sulfuric acid plant specifically to support an alkylation unit has a significant impact on both the initial requirements for capital and ongoing costs of operation.
HF alky units are also capable of processing a wider range of light-end feedstocks with propylenes and butylenes, and produce alkylate with a higher octane rating than sulfuric plants.
Solid alkylation catalyst technology was first commercialized on August 18, 2015, with the successful start-up of an alky unit at the Wonfull Refinery in Shandong Province, China.
The unit uses the AlkyClean® process technology jointly developed by Albemarle Corporation, CB&I and Neste Oil, and has a capacity of 2,700 barrels per stream day of alkylate production.
[13] The olefin feed to an alkylation unit generally originates from a FCCU and contains butene, isobutene, and possibly propene and/or amylenes.
Incondensable are from a chemical perspective similar to diluents but they do not condense at the pressure and temperature of the process, and therefore they concentrate to a point that must be vented.
High purity feedstock (> 95% vol isobutane) normally originates from an external De-isobutanizer (DIB) tower and is fed directly to the alkylation unit reaction zone.
As with the previously described mechanisms, the heavy carbocations may at some point undergo a hydride transfer from isobutane to yield a C12 – C16 isoparaffin and a t-butyl cation.
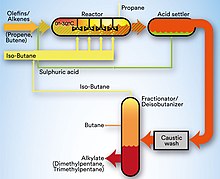
The purpose of the unit is to react an olefin feed with isobutane in the reaction section in the presence of the HF acting as catalyst to produce alkylate.
Prior to entering the reaction section, the olefin and isobutane feed are treated in a coalescer to remove water, sulfur and other contaminants.
Although they do not participate directly in the reactions, and adversely impact product quality, they provide an avenue for organic fluorides to leave the unit.
In the reaction section the reacting hydrocarbons (olefin feed with both fresh and recycled isobutane) are brought into contact with sulfuric acid catalyst under controlled conditions and at a temperature of 15.6 °C (60 °F).
Feeds that contain high amounts of propylene have a much higher rate of increase in acid consumption over the normal spending range.
When concentrations is too low catalyst activity is substantially decreased and polymerization enhanced to the point that it is difficult to maintain acid strength.
In SAAU recent studies have found that both butylenes and amylenes can be spent to a lower acid concentration without entering into a runaway condition.
In general, higher olefin space velocities tend to increase sulfuric acid consumption rates and decrease alkylate octane.
However, equal costs are realized in the HF unit by the need for feed driers, product treating, regeneration equipment and more exotic metallurgy.
The transportation of specific types of LPG streams can be expensive so local disparities in economic conditions are often not fully mitigated by cross market movements of alkylation feedstocks.
The economics of the international and local market of gasolines dictates the spread that a buyer need to pay for isobutane compared to standard commercial butane.
Other HFAU and most of SAAU develop conditions of mixing and recycle optimization such that they produce similar octane products with isobutane to olefin ratios on the order of 7 - 9/1.
Nevertheless, independent provider of energy and petrochemicals information like Platts reports trades for alkylate ready for blending in the gasoline pool, with RVP < 5.5 psi, (RON + MON)/2 > 92 and of course free of aromatics, olefins and sulfur.
In light of this high risk, the American Petroleum Institute has issued a Recommended Practice specifically for HF alkylation units (API RP 751).
The choice between these two options is site specific and usually depends on capital versus operating cost considerations and the proximity of the refinery to an existing commercial regeneration plant.
[22] Despite significant advances in process technology, there continue to be recurring corrosion problems that affect the safety and reliability of HFAU.
For this reason, many refiners utilize a weak caustic "heel" of water in the bottom of their alkylate tanks, in order to neutralize any acid that may form.