Carbocation
[3] In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom.
[5] According to the IUPAC, a carbocation is any cation containing an even number of electrons in which a significant portion of the positive charge resides on a carbon atom.
In this usage, 2-norbornyl cation is not a carbonium ion, because it is formally derived from protonation of an alkene (norbornene) rather than an alkane, although it is a non-classical carbocation due to its bridged structure.
Because of the subtle differences in the expected behavior of a non-classical carbenium ions compared to the alternative hypothesis of two rapidly equilibrating classical structures, a lively and often acrimonious debate took place over several decades regarding the merits of each model.
Therefore, carbenium ions (and carbocations in general) are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge.
Although classical carbenium ions have a structure that corresponds to a non-bridging Lewis structure, it is important to note that donation of electron density from neighboring C–H or C–C bonds into the "empty" p orbital, known as hyperconjugation, is still an important stabilizing factor, and these bonds have a tendency to "lean" towards the carbocationic center to improve orbital overlap.
The history of carbocations dates back to 1891 when G. Merling[8] reported that he added bromine to tropylidene (cycloheptatriene) and then heated the product to obtain a crystalline, water-soluble material, C7H7Br.
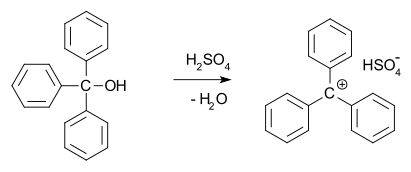
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol gives deep-yellow solutions in concentrated sulfuric acid.
The stable 7-norbornadienyl cation was prepared by Story et al. in 1960[15] by reacting norbornadienyl chloride with silver tetrafluoroborate in sulfur dioxide at −80 °C.
The NMR spectrum of the norbornyl cation was reported by Schleyer et al.[16] It was shown to rapidly undergo proton-scrambling .