Catalytic reforming
[1] The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking.
A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.
In addition to a gasoline blending stock, reformate is the main source of aromatic bulk chemicals such as benzene, toluene, xylene and ethylbenzene, which have diverse uses, most importantly as raw materials for conversion into plastics.
In the 1940s, Vladimir Haensel,[2] a research chemist working for Universal Oil Products (UOP), developed a catalytic reforming process using a catalyst containing platinum.
Today, the large majority of gasoline produced worldwide is derived from the catalytic reforming process.
The overhead liquid distillate is called naphtha and will become a major component of the refinery's gasoline (petrol) product after it is further processed through a catalytic hydrodesulfurizer to remove sulfur-containing hydrocarbons and a catalytic reformer to reform its hydrocarbon molecules into more complex molecules with a higher octane rating value.
The table just below lists some fairly typical straight-run heavy naphtha feedstocks, available for catalytic reforming, derived from various crude oils.
However, for the most part, catalytic reforming is mainly used on the straight-run heavy naphthas, such as those in the above table, derived from the distillation of crude oils.
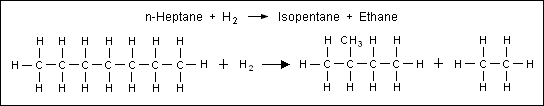
[11] The four major catalytic reforming reactions are:[12][page needed] The dehydrogenation of naphthenes to convert them into aromatics as exemplified in the conversion methylcyclohexane (a naphthene) to toluene (an aromatic): The isomerization of normal paraffins to isoparaffins as exemplified in the conversion of normal octane to 2,5-dimethylhexane (an "isoparaffin"): The dehydrogenation and aromatization of paraffins to aromatics (commonly called dehydrocyclization) as exemplified in the conversion of normal heptane to toluene: The hydrocracking of paraffins into smaller molecules as exemplified by the cracking of normal heptane into isopentane and ethane: During the reforming reactions, the carbon number of the reactants remains unchanged, except for hydrocracking reactions which break down the hydrocarbons.
[citation needed] The process flow diagram below depicts a typical semi-regenerative catalytic reforming unit.
The liquid feed (at the bottom left in the diagram) is pumped up to the reaction pressure (5–45 atm) and is joined by a stream of hydrogen-rich recycle gas.
That offgas is routed to the refinery's central gas processing plant for removal and recovery of propane and butane.
The heavy reformate is high in octane and low in benzene, hence it is an excellent blending component for the gasoline pool.
Therefore, the naphtha feedstock to a catalytic reformer is always pre-processed in a hydrodesulfurization unit which removes both the sulfur and the nitrogen compounds.
The activity (i.e., effectiveness) of the catalyst in a semi-regenerative catalytic reformer is reduced over time during operation by carbonaceous coke deposition and chloride loss.
The activity of the catalyst can be periodically regenerated or restored by in situ high temperature oxidation of the coke followed by chlorination.
However, independently of the crude oil used in the refinery, all catalysts require a maximum final boiling point of the naphtha feedstock of 180 °C.
Normally, the catalyst can be regenerated perhaps 3 or 4 times before it must be returned to the manufacturer for reclamation of the valuable platinum and/or rhenium content.
Dehydrogenation, an important component of reforming, is a strongly endothermic reaction, and as such, requires the reactor vessel to be externally heated.
This is driving the emergence of new technologies to process naphtha into gasoline by companies like Chevron Phillips Chemical (Aromax [16][failed verification] and NGT Synthesis (Methaforming,[16][17]).