Alkyne trimerisation
Trimerisation of acetylene to benzene is highly exergonic, proceeding with a free energy change of 142 kcal/mol at room temperature.
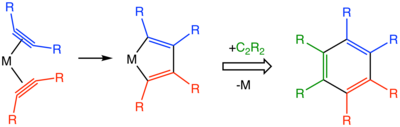
[5] Starting from the metallacyclopentadiene intermediate, many pathways can be considered including metallocycloheptatrienes, metallanorbornadienes, and a more complicated structure featuring a carbenoid ligand.
The substitution pattern about the product arene is determined in two steps: formation of the metallocyclopentadiene intermediate and incorporation of the third equivalent of alkyne.
Steric bulk on the alkyne coupling partners and catalyst have been invoked as the controlling elements of regioselectivity.
[8] In addition to high-order polymers and dimers and trimers, which originate from low regio- and chemoselectivities, enyne side products derived from alkyne dimerisation have been observed.
In one example remarkable for the formation of three new aromatic rings in one step, the triyne shown is transformed into the helical product via treatment with cyclopentadienylcobalt dicarbonyl.
[clarification needed] Using commercially available cyclopentadienylcobalt dicarbonyl, CpCo(CO)2, as catalyst, bis(trimethylsilyl)acetylene (BTMSA) will react with a diyne-1,2-disubstituted benzene to form an anthroquinone aromatic system:[14] Benzyne, generated in situ from a benzene ring bearing ortho-distributed triflate and trimethylsilyl substituents, can be used to generate an aryne in place of an acetylene and combined with a suitable diyne.
[19] Cyclotrimerization presents an alternative to the functionalization of pre-formed aromatic compounds through electrophilic or nucleophilic substitution, the regioselectivity of which can sometimes be difficult to control.
[21] Cyclization of transient benzyne species with alkynes, catalyzed by palladium, can also produce substituted aromatic compounds.