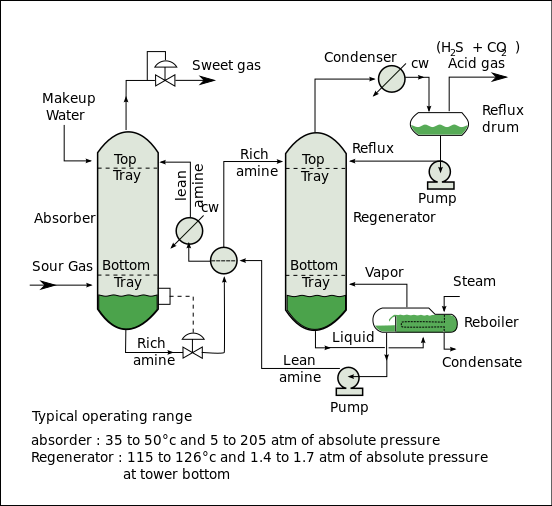
Amine gas treating
However, membrane separation is less attractive due to the relatively high capital and operating costs as well as other technical factors.
These amines are also used in many oil refineries to remove sour gases from liquid hydrocarbons such as liquified petroleum gas (LPG).
Solvents with high heats of absorption require less energy for stripping from temperature swing at fixed capacity.
An Internal Exchange stripper has a smaller ratio of water vapor to CO2 in the overhead stream, and therefore less steam is required.
When treating gases with a high percentage of CO2, corrosion inhibitors are often used and that permits the use of higher concentrations of amine in the circulating solution.
[6] MEA and DEA also require a large amount of energy to strip the CO2 during regeneration, which can be up to 70% of total operating costs.
In the specific case of the industrial synthesis of ammonia, for the steam reforming process of hydrocarbons to produce gaseous hydrogen, amine treating is one of the commonly used processes for removing excess carbon dioxide in the final purification of the gaseous hydrogen.
The removal of the sometimes high content of hydrogen sulfide is necessary to prevent corrosion of metallic parts after burning the bio gas.
For example, monoethanolamine (MEA) reacts strongly with acid gases like CO2 and has a fast reaction time and an ability to remove high percentages of CO2, even at the low CO2 concentrations.
[11] Challenges of carbon capture using amine include: The partial pressure is the driving force to transfer CO2 into the liquid phase.
Under low pressure, this transfer is hard to achieve without increasing the reboilers' heat duty, which will result in higher costs.
[12] Currently, a variety of amine mixtures are being synthesized and tested to achieve a more desirable set of overall properties for use in CO2 capture systems.
For example, the energy required for regeneration is typically related to the driving forces for achieving high capture capacities.