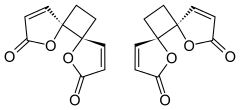
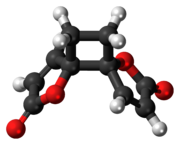
Anemonin
Anemonin is a tri-spirocyclic dibutenolide natural product found in members of the buttercup family (Ranunculaceae) such as Ranunculus bulbosus, R. ficaria, R. sardous, R. sceleratus,[2] and Clematis hirsutissima.
[4] Despite multiple possibilities, X-ray crystallography of the solid anemonin has revealed that the two rings exclusively possess a trans relationship.
[9] The highly selective formation of the head-to-head dimer has been rationalized through the stability of a proposed diradical intermediate; the resulting radicals after an initial carbon-carbon bond forming step are delocalized through the α,β-unsaturated system.
It is not mentioned whether light was excluded from this control reaction; the low yield of anemonin may arise from visible light-mediated dimerization of protoanemonin.
[17] Given its skin permeability in ethanolic solutions[18] and its anti-inflammatory and anti-pigmentation properties, anemonin may be a good candidate for topical formulations as arthritis medications or cosmetics.