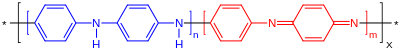
Polyaniline
[2][3] Polyaniline was discovered in the 19th century by F. Ferdinand Runge (1794–1867), Carl Fritzsche (1808–1871), John Lightfoot (1831–1872), and Henry Letheby (1816–1876).
[5][6] The first definitive report of polyaniline did not occur until 1862, which included an electrochemical method for the determination of small quantities of aniline.
[9] Polyaniline sensors typically exploit changes in electrical conductivity between the different oxidation states or doping levels.
(Per)nigraniline is prepared by oxidation of the emeraldine base with a peracid:[13] The synthesis of polyaniline nanostructures is facile.
In the second stage pernigraniline is reduced to the emeraldine salt as aniline monomer gets oxidized to the radical cation.
[17] The major applications are printed circuit board manufacturing: final finishes, used in millions of m2 every year, antistatic and ESD coatings, and corrosion protection.
[5][17] Polyaniline and its derivatives are also used as the precursor for the production of N-doped carbon materials through high-temperature heat treatment.