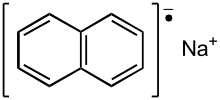
Radical anion
Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide.
The electron is transferred from the alkali metal ion to an unoccupied antibonding p-p п* orbital of the aromatic molecule.
This transfer is usually only energetically favorable if the aprotic solvent efficiently solvates the alkali metal ion.
Effective solvents are those that bind to the alkali metal cation: diethyl ether < THF < 1,2-dimethoxyethane < HMPA.
In principle any unsaturated molecule can form a radical anion, but the antibonding orbitals are only energetically accessible in more extensive conjugated systems.
Radical anions of polycyclic aromatic compounds function as ligands in organometallic chemistry.
[10] Radical cations figure prominently in the chemistry and properties of conducting polymers.
[11] Polarons and bipolarons are radical cations encountered in doped conducting polymers.