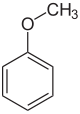
Anisole
It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances.
[2] Anisole undergoes electrophilic aromatic substitution reaction at a faster speed than benzene, which in turn reacts more quickly than nitrobenzene.
The enhanced nucleophilicity of anisole vs. benzene reflects the influence of the methoxy group, which renders the ring more electron-rich.
Illustrative of its nucleophilicity, anisole reacts with acetic anhydride to give 4-methoxyacetophenone: Unlike most acetophenones, but reflecting the influence of the methoxy group, methoxyacetophenone undergoes a second acetylation.
[6] Anisole was first synthesized in 1841 by Auguste Cahours by barium anisate decarboxylation while heating p-anisic acid he made earlier from the anise essence with barium oxide:[7][8] 2 CH3OC6H4COOH + BaO → (CH3OC6H4COO)2Ba + H2O (CH3OC6H4COO)2Ba → 2 CH3OC6H5 + BaCO3 It can be prepared by the Williamson ether synthesis from sodium phenoxide and dimethyl sulfate or methyl chloride:[9][5] Anisole is a precursor to perfumes, insect pheromones, and pharmaceuticals.