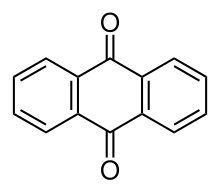
Anthraquinone
Several isomers exist but these terms usually refer to 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the keto groups are located on the central ring.
Many anthraquinone derivatives are generated by organisms or synthesised industrially for use as dyes, pharmaceuticals, and catalysts.
[7] The anthraquinone oxidizes the reducing end of polysaccharides in the pulp, i.e., cellulose and hemicellulose, and thereby protecting it from alkaline degradation (peeling).
This process gives an increase in yield of pulp, typically 1–3% and a reduction in kappa number.
[10] It has also been mixed with lanolin and used as a wool spray to protect sheep flocks against kea attacks in New Zealand.