Apicomplexa
The Apicomplexa (also called Apicomplexia; single: apicomplexan) are organisms of a large phylum of mainly parasitic alveolates.
Most possess a unique form of organelle structure that comprises a type of non-photosynthetic plastid called an apicoplast—with an apical complex membrane.
The organelle's apical shape (e.g., see Ceratium furca) is an adaptation that the apicomplexan applies in penetrating a host cell.
The Apicomplexa are a diverse group that includes organisms such as the coccidia, gregarines, piroplasms, haemogregarines, and plasmodia.
The Apicomplexa comprise the bulk of what used to be called the Sporozoa, a group of parasitic protozoans, in general without flagella, cilia, or pseudopods.
The phylum Apicomplexa contains all eukaryotes with a group of structures and organelles collectively termed the apical complex.
[7] This complex consists of structural components and secretory organelles required for invasion of host cells during the parasitic stages of the Apicomplexan life cycle.
[7] Apicomplexa have complex life cycles, involving several stages and typically undergoing both asexual and sexual replication.
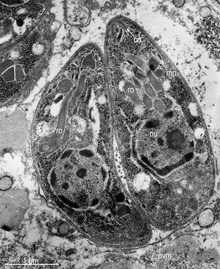
The apical complex consists of a set of spirally arranged microtubules (the conoid), a secretory body (the rhoptry) and one or more polar rings.
Additional slender electron-dense secretory bodies (micronemes) surrounded by one or two polar rings may also be present.
A further group of spherical organelles is distributed throughout the cell rather than being localized at the apical complex and are known as the dense granules.
Secretion of the dense-granule content takes place after parasite invasion and localization within the parasitophorous vacuole and persists for several minutes.
[citation needed] Replication: Mobility: Apicomplexans have a unique gliding capability which enables them to cross through tissues and enter and leave their host cells.
[9] Other features common to this phylum are a lack of cilia, sexual reproduction, use of micropores for feeding, and the production of oocysts containing sporozoites as the infective form.
Typically, a host is infected via an active invasion by the parasites (similar to entosis), which divide to produce sporozoites that enter its cells.
[11] The apical complex includes vesicles called rhoptries and micronemes, which open at the anterior of the cell.
The ookinete then transforms into an oocyst and divides initially by meiosis and then by mitosis (haplontic lifecycle) to give rise to the sporozoites.
[21] The class Marosporida Mathur, Kristmundsson, Gestal, Freeman, and Keeling 2020 is a newly recognized lineage of apicomplexans that is sister to the Coccidia and Hematozoa.
It is defined as a phylogenetic clade containing Aggregata octopiana Frenzel 1885, Merocystis kathae Dakin, 1911 (both Aggregatidae, originally coccidians), Rhytidocystis sp.
However, marosporidians have the most reduced apicoplast genomes sequenced to date, lack canonical plastidial RNA polymerase and so provide new insights into reductive organelle evolution.
In contrast to bacterial pathogens, these apicomplexan parasites are eukaryotic and share many metabolic pathways with their animal hosts.
The availability of genome sequences provides a new opportunity for scientists to learn more about the evolution and biochemical capacity of these parasites.
The male gametocyte produces a large number of gametes and the zygote gives rise to an oocyst, which is the infective stage.
The first Apicomplexa protozoan was seen by Antonie van Leeuwenhoek, who in 1674 saw probably oocysts of Eimeria stiedae in the gall bladder of a rabbit.
[32] However, other parasitic or symbiotic unicellular organisms were included too in protozoan groups outside Sporozoa (Flagellata, Ciliophora and Sarcodina), if they had flagella (e.g., many Kinetoplastida, Retortamonadida, Diplomonadida, Trichomonadida, Hypermastigida), cilia (e.g., Balantidium) or pseudopods (e.g., Entamoeba, Acanthamoeba, Naegleria).
The division into Achromatorida and Chromatorida, although proposed on morphological grounds, may have a biological basis, as the ability to store haemozoin appears to have evolved only once.
[40] Roberts and Janovy in 1996 divided the phylum into the following subclasses and suborders (omitting classes and orders):[41] These form the following five taxonomic groups: Perkins et al. proposed the following scheme.
[42] It is outdated as the Perkinsidae have since been recognised as a sister group to the dinoflagellates rather that the Apicomplexia: The name Protospiromonadida has been proposed for the common ancestor of the Gregarinomorpha and Coccidiomorpha.
[16] Squirmida (Digyalum, Filipodium, Platyproteum) Chromerida (Chromera, Vitrella, Piridium) Colpodellida (Colpodella) Voromonadida (Alphamonas, Voromonas) Cryptosporidium Gregarines s.s. Aggregatidae (Aggregata, Merocystis) Margolisiella Rhytidocystidae (Rhytidocystis) Hemogregarines Coccidia with a single host (Eimeria, Isospora, Cyclospora) Cyst-forming coccidia (Toxoplasma, Sarcocystis, Frenkellia) Piroplasms (Babesia, Theileria) Hemosporidia (Plasmodium, Leucocytozoon) Dinoflagellates & Perkinsozoa Janouškovec et al. 2015 presents a somewhat different phylogeny, supporting the work of others showing multiple events of plastids losing photosynthesis.


- Anterior polar ring
- Intra-conoid microtubules
- Conoid
- Posterior polar ring
- Inner membrane complex
- Subpellicular microtubules
- Rhoptries , hold enzymes released during host penetration
- Micronemes , important for host-cell invasion and gliding motility
- Mitochondrion , creates ATP (energy) for the cell (tubular cristae)
- Micropore
- Dense granules
- Apicoplast membranes (4, secondary red, non-photosynthetic)
- Golgi apparatus ; modifies proteins and sends them out of the cell
- Nucleus
- Endoplasmic reticulum , the transport network for molecules going to specific parts of the cell