Microtubule
[4] The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.Microtubules play an important role in a number of cellular processes.
[5] They are also involved in cell division (by mitosis and meiosis) and are the main constituents of mitotic spindles, which are used to pull eukaryotic chromosomes apart.
[6] Recently an actin-like protein has been found in the gram-positive bacterium Bacillus thuringiensis, which forms a microtubule-like structure called a nanotubule, involved in plasmid segregation.
The α and β-tubulin subunits are ~50% identical at the amino acid level, and both have a molecular weight of approximately 50 kDa.
[19] There are two distinct types of interactions that can occur between the subunits of lateral protofilaments within the microtubule called the A-type and B-type lattices.
These long chains (protofilaments) now gradually accumulate next to each other so that a tube-like structure is formed, which has a lumen typical of a tube.
The roles of the microtubule cytoskeleton include mechanical support, organization of the cytoplasm, transport, motility and chromosome segregation.
In epithelia, the minus-ends of the microtubule polymer are anchored near the site of cell-cell contact and organized along the apical-basal axis.
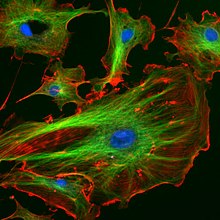
In fibroblasts and other mesenchymal cell-types, microtubules are anchored at the centrosome and radiate with their plus-ends outwards towards the cell periphery (as shown in the first figure).
Moreover, the polarity of microtubules is acted upon by motor proteins, which organize many components of the cell, including the endoplasmic reticulum and the Golgi apparatus.
In addition, work from the Kaverina group at Vanderbilt, as well as others, suggests that the Golgi apparatus can serve as an important platform for the nucleation of microtubules.
In recent studies, the Vale group at UCSF identified the protein complex augmin as a critical factor for centrosome-dependent, spindle-based microtubule generation.
[34] In 1986, Marc Kirschner and Tim Mitchison proposed that microtubules use their dynamic properties of growth and shrinkage at their plus ends to probe the three dimensional space of the cell.
Suppression of microtubule dynamics by tubulin mutations or by drug treatment have been shown to inhibit cell migration.
In general the dynamics are normally suppressed by low, subtoxic concentrations of microtubule drugs that also inhibit cell migration.
However, incorporating β3-tubulin into microtubules increases the concentration of drug that is needed to suppress dynamics and inhibit cell migration.
Very low levels of free calcium can destabilize microtubules and this prevented early researchers from studying the polymer in vitro.
The rates of microtubule polymerization, depolymerization, and catastrophe vary depending on which microtubule-associated proteins (MAPs) are present.
In-vitro, tau proteins have been shown to directly bind microtubules, promote nucleation and prevent disassembly, and to induce the formation of parallel arrays.
Three proteins called katanin, spastin, and fidgetin have been observed to regulate the number and length of microtubules via their destabilizing activities.
The first MAP to be identified as a +TIP was CLIP170 (cytoplasmic linker protein), which has been shown to play a role in microtubule depolymerization rescue events.
[citation needed] Microtubules can act as substrates for motor proteins that are involved in important cellular functions such as vesicle trafficking and cell division.
Some viruses (including retroviruses, herpesviruses, parvoviruses, and adenoviruses) that require access to the nucleus to replicate their genomes attach to motor proteins.
Once there they interact with specific motor proteins which create force that pull the microtubules, and thus the entire centrosome towards the cell membrane.
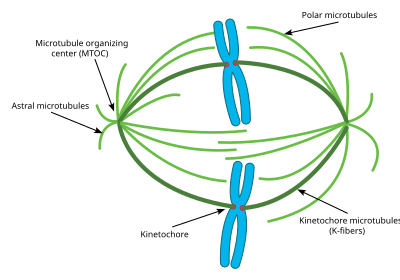
Experiments have shown that without these astral microtubules, the mitotic spindle can form, however its orientation in the cell is not always correct and thus mitosis does not occur as effectively.
Each K fiber is composed of 20–40 parallel microtubules, forming a strong tube which is attached at one end to the centrosome and on the other to the kinetochore, located in the center of each chromosome.
[72] It has been found that microtubules act as "struts" that counteract the contractile forces that are needed for trailing edge retraction during cell movement.
The action of the dynein motor proteins on the various microtubule strands that run along a cilium or flagellum allows the organelle to bend and generate force for swimming, moving extracellular material, and other roles.
For example, a network of polarized microtubules is required within the oocyte of Drosophila melanogaster during its embryogenesis in order to establish the axis of the egg.
[76] The cellular cytoskeleton is a dynamic system that functions on many different levels: In addition to giving the cell a particular form and supporting the transport of vesicles and organelles, it can also influence gene expression.