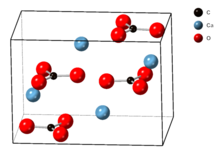
Aragonite
[6] The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797.
[14] Because the mineral deposition in mollusk shells is strongly biologically controlled,[15] some crystal forms are distinctively different from those of inorganic aragonite.
[20][21] Aragonite also forms in the ocean inorganic precipitates called marine cements (in the sediment) or as free crystals (in the water column).
The weak Van der Waals forces inside aragonite give an important contribution to both the crystallographic and elastic properties of this mineral.
[29] The difference in stability between aragonite and calcite, as measured by the Gibbs free energy of formation, is small, and effects of grain size and impurities can be important.
[32] The mineral vaterite, also known as μ-CaCO3, is another phase of calcium carbonate that is metastable at ambient conditions typical of Earth's surface, and decomposes even more readily than aragonite.
Aragonite provides the materials necessary for much sea life and also keeps the pH of the water close to its natural level, to prevent the dissolution of biogenic calcium carbonate.