Asymmetric cell division
The cell biology of these events has been most studied in three animal models: the mouse, the nematode Caenorhabditis elegans, and the fruit fly Drosophila melanogaster.
In C. elegans, a series of asymmetric cell divisions in the early embryo are critical in setting up the anterior/posterior, dorsal/ventral, and left/right axes of the body plan.
This series of events allows the single celled zygote to obtain polarity through an unequal distribution of multiple factors.
[10] In Drosophila melanogaster, asymmetric cell division plays an important role in neural development.
The neuroblast repeatedly undergoes this asymmetric cell division while the GMC continues on to produce a pair of neurons.
Two proteins play an important role in setting up this cell fate asymmetry in the neuroblast, Prospero and Numb.
Without the presence of Inscuteable, the positioning of the mitotic spindle and the cell fate determinants in relationship to each other becomes randomized.
[15][16] The mechanism instead relies on the spatial and temporal organization of myosin on the cell cortex and its upstream components.
[19][20] This spatiotemporal control of myosin localization results in the asymmetric loss of cortical tension that normally pushes against hydrostatic pressure.
Although much is known at the cellular and molecular level about the other bilateralian clades (ecdysozoa and deuterostomia), research into the processes that govern spiralian development is comparatively lacking.
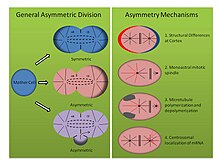
[25] Mechanisms of asymmetric division (See Figure, right panel): Animals are made up of a vast number of distinct cell types.
Intrinsic factors generally involve differing amounts of cell-fate determinants being distributed into each daughter cell.
[29] In addition to the aforementioned Drosophila neuronal example, it was proposed that the macrosensory organs of the Drosophila, specifically the glial cells, also arise from a similar set of asymmetric division from a single progenitor cell via regulation of the Notch signaling pathway and transcription factors.
In normal non-tumor stem cells, a number of genes have been described which are responsible for pluripotency, such as Bmi-1, Wnt and Notch.
The asymmetric division in these cells is regulated by cancer niche (microenvironment) and Wnt pathway.
Studies of loss-of-function mutations in key regulators of asymmetric cell division including lgl, aurA, polo, numb and brat, revealed hyperproliferative phenotypes in situ.