Cell cortex
Due to the branching process and the density of the actin cortex, the cortical cytoskeleton can comprise a highly complex meshwork such as a fractal structure.
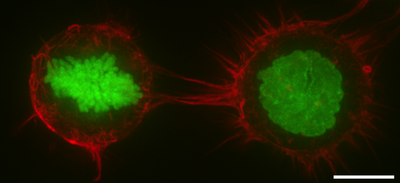
[12] In mitosis, F-actin and myosin II form a highly contractile and uniform cortex to drive mitotic cell rounding.
The surface tension produced by the actomyosin cortex activity generates intracellular hydrostatic pressure capable of displacing surrounding objects to facilitate rounding.
[16][17] Genetic studies have shown that the cell cortex in mitosis is regulated by diverse genes such as Rhoa,[18] WDR1,[19] ERM proteins,[20] Ect2,[21] Pbl, Cdc42, aPKC, Par6,[22] DJ-1 and FAM134A.
By pulling on adhesion complexes, the cortex promotes the expansion of contacts with other cells or with the extracellular matrix.
Notably, during early mammalian development, the cortex pulls cells together to drive compaction and the formation of the morula.
[26][27] Also, differences in cortical tension drives the sorting of the inner cell mass and trophectoderm progenitors during the formation of the morula,[28] the sorting of germ layer progenitors during zebrafish gastrulation,[29][30] the invagination of the mesoderm and the elongation of the germ band elongation during drosophila gastrulation.