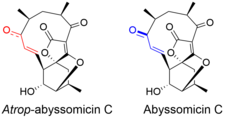
Atrop-abyssomicin C
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin.
The molecule displays antibacterial activity by inhibiting the enzyme PabB (4-amino-4-deoxychorismate synthase), thereby depleting the biosynthesis of p-aminobenzoate.
[5] Despite being a strained macrocycle, there exist an atropisomer, abyssomicin C. The atropisomerism arise due to a structural deviation in the α,β-unsaturated ketone region of the molecule.
[7] The biosynthesis of atrop-abyssomicin C begins with the synthesis of a linear polyketide chain in a PKS I system that consist of one loading and six extension modules.
[1] An intramolecular Diels-Alder was proposed to take place between the exocyclic olefin and the conjugated diene at the tail end of the polyketide to form the macrocyclic ring.