Auwers synthesis
The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone.
This reaction was first reported by Karl von Auwers in 1908.
[1][2][3][4][5] The first step in this procedure is an acid catalyzed aldol condensation between benzaldehyde and a 3-cyclooxapentanone to an o-hydroxychalcone.
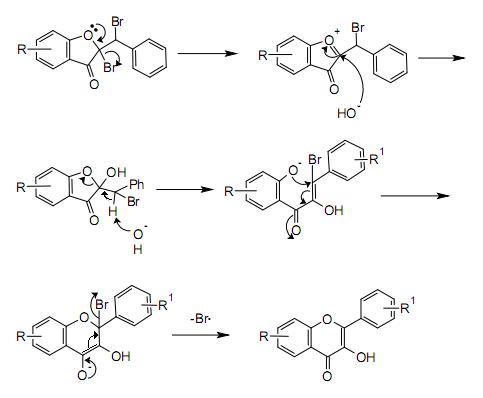
Bromination of the alkene group gives a dibromo-adduct which rearranges to the flavonol by reaction with potassium hydroxide.
A possible mechanism for the rearrangement step is shown below: