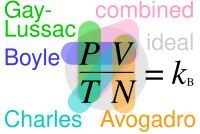
Avogadro's law
A modern statement is: Avogadro's law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules.
The law is named after Amedeo Avogadro who, in 1812,[2][3] hypothesized that two given samples of an ideal gas, of the same volume and at the same temperature and pressure, contain the same number of molecules.
As an example, equal volumes of gaseous hydrogen and nitrogen contain the same number of molecules when they are at the same temperature and pressure, and display ideal gas behavior.
The hypothesis was first published by Amedeo Avogadro in 1811,[4] and it reconciled Dalton atomic theory with the "incompatible" idea of Joseph Louis Gay-Lussac that some gases were composite of different fundamental substances (molecules) in integer proportions.
[7] Experimental studies carried out by Charles Frédéric Gerhardt and Auguste Laurent on organic chemistry demonstrated that Avogadro's law explained why the same quantities of molecules in a gas have the same volume.
This apparent contradiction was finally resolved by Stanislao Cannizzaro, as announced at Karlsruhe Congress in 1860, four years after Avogadro's death.