Azetidine
Azetidines can be prepared by reduction of azetidinones (β-lactams) with lithium aluminium hydride.
[4] Regio- and diastereoselective synthesis of 2-arylazetidines could be performed from appropriately substituted oxiranes via ring transformation.
It is controlled by Baldwin's Rules with remarkable functional group tolerance.
[citation needed] Azetidine and its derivatives are relatively rare structural motifs in natural products.
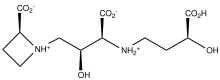
Perhaps the most abundant azetidine containing natural product is azetidine-2-carboxylic acid - a toxic mimic of proline.