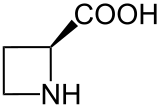
Azetidine-2-carboxylic acid
Optically inactive Aze was obtained in small yield from the neurotransmitter GABA by α-bromination, followed by removal of hydrogen bromide from the intermediate γ-amino-α-bromobutyric acid and ring closure by treatment with a barium hydroxide solution.
It is known to occur in two species from the Asparagaceae - Convallaria majalis (lily of the valley), and Polygonatum (solomon's seal).
Other studies have shown effects of Aze resulting in a wide range of toxic and teratogenic disorders, including in a range of malformations, in various animal species including ducks, hamsters, mice, and rabbits.
[2] Molecular studies of human prolyl- and alanyl-tRNA synthetases suggest that Aze is incorporated in proteins as proline with toxic consequences in vivo.
[6] Even if Aze seems to fit into the active site of both tRNA synthetases (due to its double mimicry effect of alanine and proline), it is rejected by alanyl-tRNA synthetases post-transfer editing system.