Bacterial circadian rhythm
In these bacteria, three key proteins whose structures have been determined, KaiA, KaiB, and KaiC can form a molecular clockwork that orchestrates global gene expression.
[2] In 1985–6, several research groups discovered that cyanobacteria display daily rhythms of nitrogen fixation in both light/dark (LD) cycles and in constant light.
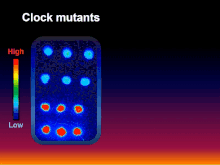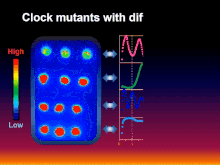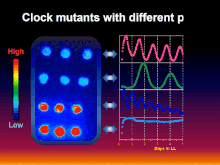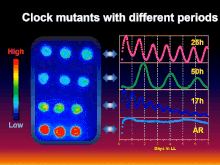
[5] Inspired by the research of the aforementioned pioneers, the collaborative group of Takao Kondo, Carl H. Johnson, Susan Golden, and Masahiro Ishiura genetically transformed the cyanobacterium Synechococcus elongatus with a luciferase reporter that allowed rhythmic gene expression to be assayed non-invasively as rhythmically "glowing" cells.
Circadian clocks are assumed to enhance the fitness of organisms by improving their ability to predict and anticipate daily cycles in environmental factors.
The idea was to determine if having an appropriately functional clock system enhances fitness under competitive conditions.
[28] In vivo, the output of this biochemical KaiABC oscillator to rhythms of gene expression appears to be mediated by KaiC phosphorylation status (see below) regulating a biochemical cascade involving a histidine kinase SasA and a phosphatase CikA that activate/inactivate the globally acting transcription factor RpaA.
KaiC forms a hexamer that resembles a double doughnut with a central pore that is partially sealed at one end.
[35][36] The three-dimensional structures have been helpful in elucidating the cyanobacterial clock mechanism by providing concrete models for the ways in which the three Kai proteins interact and influence each other.
[30][34][35][37][38][39][40] The structural approaches have also allowed the KaiA/KaiB/KaiC complexes to be visualized as a function of time, which enabled sophisticated mathematical modeling of the in vitro phosphorylation rhythm.
In addition, single-molecule methods (high-speed atomic force microscopy) have been applied to visualize in real time and quantify the dynamic interactions of KaiA with KaiC on sub-second timescales.
Phosphorylation/dephosphorylation reactions and protein complex associations/dissassociations can be very rapid, so why does this biochemical oscillator have a period that is as slow as 24 hours and yet still be so precise?
Therefore, some authors have proposed that the KaiC ATPase activity constitutes the most fundamental reaction underlying circadian periodicity in cyanobacteria.
[43] The purple non-sulfur bacterium Rhodopseudomonas palustris is one such example, as it harbors homologs of KaiB and KaiC and exhibits adaptive KaiC-dependent growth enhancement in 24-hour cyclic environments.
[49] However, R. palustris was reported to show a poor intrinsic free-running rhythm of nitrogen fixation under constant conditions.
The lack of rhythm in R. palustris in constant conditions has implications for the adaptive value of intrinsic timekeeping mechanism.
A long-term study on mice was conducted to determine whether the hosts’ rhythmic and arrhythmic feeding behaviors contributed differently to the recoveries of their gut microbiota from antibiotic treatment.
[58] Researchers found that rhythmic behavior after antibiotic ablation facilitates complete recovery of the gut microbiota.
The genus Turicibacter, proven to modulate the mood-related neurotransmitter serotonin,[59] was found to overly recover.