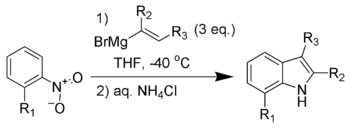
Bartoli indole synthesis
The steric bulk of the ortho group assists in the [3,3]-sigmatropic rearrangement required for product formation.
The reaction mechanism[8] of the Bartoli indole synthesis is illustrated below using o-nitrotoluene (1) and propenyl Grignard (2) to form 3,7-dimethylindole (13).
Reaction workup eliminates water and gives the final desired indole (13).
Additionally, reaction of the nitroso intermediate (4) with two equivalents of the Grignard reagent produces the expected indole.
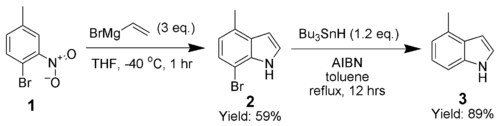
Adrian Dobbs greatly enhanced the scope of the Bartoli indole synthesis by using an ortho-bromine as a directing group, which is subsequently removed by AIBN and tributyltin hydride.