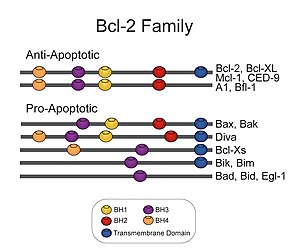
Bcl-2 family
The Bcl-2 family (TC# 1.A.21) consists of a number of evolutionarily-conserved proteins that share Bcl-2 homology (BH) domains.
Members of the BCL-2 family regulate apoptosis in mammals, reptiles, amphibs, fish, and other phyla of metazoan life, with exception of nematodes and insects.
[4] Bcl-2 family proteins have a general structure that consists of a hydrophobic α-helix surrounded by amphipathic α-helices.
The high resolution structure of the monomeric soluble form of human Bcl-x(L) has been determined by both x-ray crystallography and NMR.
Diphtheria toxin forms a transmembrane pore and translocates the toxic catalytic domain into the animal cell cytoplasm.
[8][9] Many viruses have found a way of countering defensive apoptosis by encoding their own anti-apoptosis genes preventing their target-cells from dying too soon.
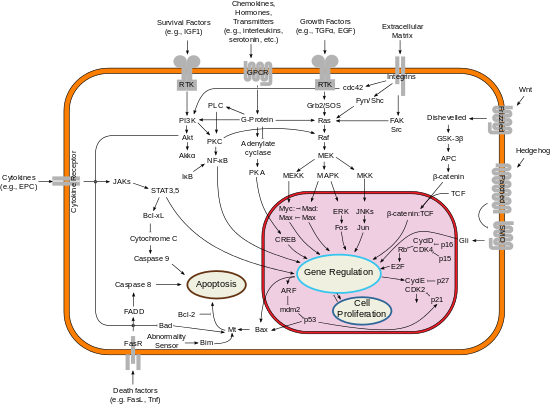
An important one states that this is achieved by activation or inactivation of an inner mitochondrial permeability transition pore, which is involved in the regulation of matrix Ca2+, pH, and voltage.
[15] These proteins are localized to the outer mitochondrial membrane of the animal cell where they are thought to form a complex with the voltage-dependent anion channel porin (VDAC).
[16] Within the mitochondria are apoptogenic factors (cytochrome c, Smac/Diablo homolog, Omi) that if released activate the executioners of apoptosis, the caspases.
Various apoptotic stimuli induce expression and/or activation of specific BH3-only family members, which translocate to the mitochondria and initiate Bax/Bak-dependent apoptosis.