Benson group increment theory
Benson group-increment theory (BGIT), group-increment theory, or Benson group additivity uses the experimentally calculated heat of formation for individual groups of atoms to calculate the entire heat of formation for a molecule under investigation.
Heats of formations are intimately related to bond-dissociation energies and thus are important in understanding chemical structure and reactivity.
In the symmetrical reaction the cleavage between the CH2 in both reactants leads to one product formation.Though difficult to see, one can see[clarify] that the neighboring carbons are not changed as the rearrangement occurs.
The methoxy and hydroxyl rearrangement display clear evidence that the neighboring groups are not affected in the disproportionation reaction.
However, they noted that under all approximations ringed systems and unsaturated centers do not follow additivity rules due to their preservation under disproportionation reactions.
As stated above, BGIT can be used to calculate heats of formation, which are important in understanding the strengths of bonds and entire molecules.
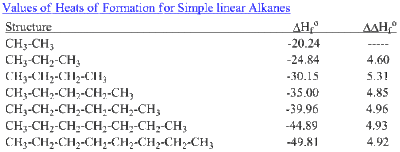
These values are for gas-phase thermodynamics and typically at 298 K. Benson and coworkers have continued collecting data since their 1958 publication and have since published even more group increments, including strained rings, radicals, halogens, and more.
[8][10][11][12] Even though BGIT was introduced in 1958 and would seem to be antiquated in the modern age of advanced computing, the theory still finds practical applications.
In a 2006 article, Gronert states: "Aside from molecular mechanics computer packages, the best known additivity scheme is Benson's.
"[13] Fishtik and Datta also give credit to BGIT: "Despite their empirical character, GA methods continue to remain a powerful and relatively accurate technique for the estimation of thermodynamic properties of the chemical species, even in the era of supercomputers"[14] When calculating the heat of formation, all the atoms in the molecule must be accounted for (hydrogen atoms are not included as specific groups).
The total calculations add to −5.15 kcal/mol (−21.6 kJ/mol), which is identical to the experimental value, which can be found in the National Institute of Standards and Technology Chemistry WebBook.
Note that these are not identically equal to the accepted strain energies for the parent ring system, although they are quite close.
In contrast, the strain energy of cyclobutane is specific to the parent compound, with their new corrections, it is now possible to predict ΔfH° values for strained ring system by first adding up all the basic group increments and then adding appropriate ring-strain correction values.
Also, the BGIT fails when conjugation and interactions between functional groups exist,[17][18] such as intermolecular and intramolecular hydrogen bonding, which limits its accuracy and usage in some cases.