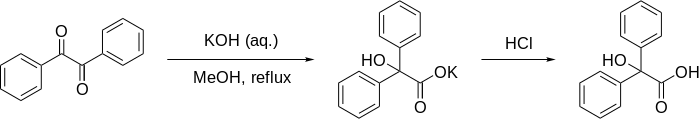
Benzilic acid rearrangement
The reaction works best when the ketone functional groups have no adjacent enolizable protons, as this allows aldol condensation to compete.
The long-established reaction mechanism was first proposed in its entirety by Christopher Kelk Ingold, and has been updated with in silico data[6] as outlined below.
Calculations show that an accurate description of the reaction sequence is possible with the participation of 4 water molecules taking responsibility for the stabilization of charge buildup.
In deuterated water, carbonyl oxygen exchange occurs much faster than the rearrangement, indicating that the first equilibrium is not the rate-determining step.
This ruled out a concerted mechanism for the reaction, as hydrogen transfer would occur in the rate determining step.
This reaction is identical to the normal benzilic acid rearrangement, except that an alkoxide or an amide anion is used in place of a hydroxide ion.
The alkoxide used should not be easily oxidizable (such as potassium ethoxide) as this favors the Meerwein–Ponndorf–Verley reduction pathway as a side reaction.