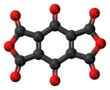
Benzoquinonetetracarboxylic dianhydride
Benzoquinonetetracarboxylic dianhydride is an organic compound with formula C10O8 (an oxide of carbon) which can be seen as the result of removing two molecules of water H2O from benzoquinonetetracarboxylic acid.
It reacts with acetone, ethyl acetate, tetrahydrofuran, ethanol, and water.
It dissolves in methylated derivatives of benzene to give solutions ranging from orange to violet.
When the molecule is exposed to moist air, it quickly turns blue.
The compound was synthesized in 1963 by P. R. Hammond, who claimed it was "one of the strongest π-electron acceptors so far described.