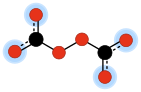
Peroxydicarbonate
Its molecular structure can be viewed as two carbonate anions joined so as to form a peroxide bridge –O–O–.
The anion is formed, together with peroxocarbonate CO2−4, at the negative electrode during electrolysis of molten lithium carbonate.
[2] In addition, the peroxodicarbonate anion can be obtained by electrosynthesis on boron doped diamond (BDD) during water oxidation.
Recent publications show that a concentration of 282 mmol/L of peroxodicarbonate can be reached in an undivided cell with sodium carbonate as starting material at current densities of 720 mA/cm2.
Potassium peroxydicarbonate K2C2O6 was obtained by Constam and von Hansen in 1895;[6] its crystal structure was determined only in 2002.