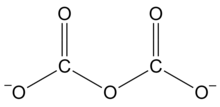
Dicarbonate
Three important organic compounds containing this group are: It is one of the oxocarbon anions, consisting solely of oxygen and carbon.
Dicarbonate salts are apparently unstable at ambient conditions, but can be made under pressure and may have a fleeting existence in carbonate solutions.
It is also sometimes used for chemicals that contain two carbonate units in their covalent structure or stoichiometric formula.
PbC2O5 (lead(II) dicarbonate) can be formed at 30 GPa and 2000K from PbCO3 and CO2.
[4] SrC2O5 (strontium dicarbonate) is very similar to the lead compound, and also has monoclinic structure with space group P21/c and four formula units per unit cell.