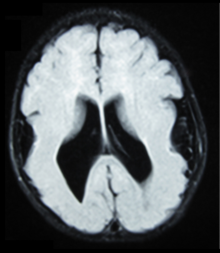
Bilateral frontoparietal polymicrogyria
Bilateral frontoparietal polymicrogyria is a genetic disorder with autosomal recessive inheritance that causes a cortical malformation.
From ongoing research, mutation in GPR56, a member of the adhesion G protein-coupled receptor (GPCR) family, results in BFPP.
However, the presence of other abnormalities in these cobblestone lissencephaly syndromes, including ocular anomalies, congenital muscular dystrophy, ventriculomegaly, and cerebellar dysplasia, usually distinguishes these disorders from polymicrogyria.
[5] There are no anatomopathologic studies that have characterized the pattern of cortical laminar alterations in patients with GPR56 gene mutations, but it has been suggested that the imaging characteristics of BFPP, including myelination defects and cerebellar cortical dysplasia, are reminiscent of those of the so-called cobblestone malformations (muscle-eye-brain disease and Fukuyama congenital muscular dystrophy) that are also associated with N-glycosylation defects in the developing brain.
[9] Diagnostic criteria for a BFPP patient entails a heterozygous genotype for a deletion of chromosome 16q12.1-q21 region, including GPR56 gene.
[12] BFPP patients demonstrate mental retardation, language impairment, motor developmental delay, and seizure disorders such as epilepsy.
In more severe forms, focal motor, sensory, visual, or cognitive problems may be present, depending on the location of the brain region affected.
These tests for example, using animals such as mice, RNAi, Behavioral assay, Electron microscopy, CT scan, or MRI demonstrate different results that concludes an affected BFPP patient.
[5] Gross neuropathologic examination reveals a pattern of complex convolutions to the cerebral cortex, with miniature gyri fused and superimposed together, often resulting in an irregular brain surface.
Genetic counseling may also be recommended[16] Once the diagnosis of polymicrogyria has been established in an individual, the following approach can be used for discussion of prognosis: A pregnancy history should be sought, with particular regard to infections, trauma, multiple gestations, and other documented problems.
The following may help in determining a genetic etiology: It is important to ask for the presence of neurologic problems in family members, including seizures, cognitive delay, motor impairment, pseudobulbar signs, and focal weakness because many affected family members, particularly those who are older, may not have had MRI performed, even if these problems came to medical attention.
In addition, although most individuals with polymicrogyria do present with neurologic difficulties in infancy, childhood, or adulthood, those with mild forms may have no obvious deficit or only minor manifestations, such as a simple lisp or isolated learning disability.
Therefore, if a familial polymicrogyria syndrome is suspected, it may be reasonable to perform MRI on relatives who are asymptomatic or have what appear to be minor findings.
A general physical examination of the proband may identify associated craniofacial, musculoskeletal, or visceral malformations that could indicate a particular syndrome.