Platelet
[1] Platelets have no cell nucleus; they are fragments of cytoplasm derived from the megakaryocytes[2] of the bone marrow or lung,[3] which then enter the circulation.
In addition to facilitating the clotting process, platelets contain cytokines and growth factors which can promote wound healing and regeneration of damaged tissues.
[14] This approximation can be used to model the hydrodynamic and optical properties of a population, as well as to restore the geometric parameters of individual measured platelets by flow cytometry.
[5] Despite much overlap, platelet function can be modeled in three steps: Thrombus formation on an intact endothelium is prevented by nitric oxide,[19] prostacyclin,[20] and CD39.
[22] The intact endothelial lining inhibits platelet activation by producing nitric oxide, endothelial-ADPase, and PGI2 (prostacyclin).
The other ADP-receptor P2Y1 couples to Gq that activates phospholipase C-beta 2 (PLCB2), resulting in inositol 1,4,5-trisphosphate (IP3) generation and intracellular release of more calcium.
These are G protein-coupled receptors and they turn on calcium-mediated signaling pathways within the platelet, overcoming the baseline calcium efflux.
This occurs by altering the metabolic flux of platelet's eicosanoid synthesis pathway, which involves enzymes phospholipase A2, cyclo-oxygenase 1, and thromboxane-A synthase.
Granule characteristics: As shown by flow cytometry and electron microscopy, the most sensitive sign of activation, when exposed to platelets using ADP, are morphological changes.
This complex runs just beneath these membranes and is the chemical motor that pulls the invaginated OCS out of the interior of the platelet, like turning pants pockets inside out, creating the dendrites.
One of the signaling pathways turns on scramblase, which moves negatively charged phospholipids from the inner to the outer platelet membrane surface.
[32] Platelets have a central role in innate immunity, initiating and participating in multiple inflammatory processes, directly binding and even destroying pathogens.
Clinical data show that many patients with serious bacterial or viral infections have thrombocytopenia, thus reducing their contribution to inflammation.
Platelet-leukocyte aggregates (PLAs) found in circulation are typical in sepsis or inflammatory bowel disease, showing the connection between thrombocytes and immune cells.
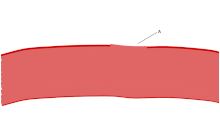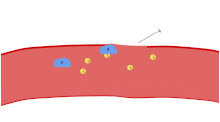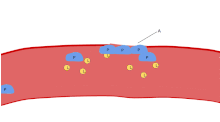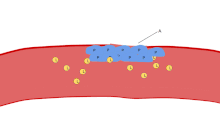
[7] In case of injury, platelets, together with the coagulation cascade, provide the first line of defense by forming a blood clot.
For example, in the Atlantic horseshoe crab (estimated to be over 400 million years old), the only blood cell type, the amebocyte, facilitates both the hemostatic function and the encapsulation and phagocytosis of pathogens by means of exocytosis of intracellular granules containing bactericidal defense molecules.
DIC in sepsis is a prime example of both the dysregulated coagulation process as well as an undue systemic inflammatory response, resulting in a multitude of microthrombi of similar composition to that in physiological immunothrombosis — fibrin, platelets, neutrophils and NETs.
Proinflammatory platelet microvesicles trigger constant cytokine secretion from neighboring fibroblast-like synoviocytes, most prominently Il-6 and Il-8.
[49] Duke's test measured the time taken for bleeding to stop from a standardized wound in the ear lobe that was blotted every 30 seconds, considering less than 3 minutes as normal.
[50] Bleeding time has low sensitivity and specificity for mild to moderate platelet disorders and is no longer recommended for screening.
These agonists induce platelet adhesion, activation and aggregation, leading to rapid occlusion of the aperture and cessation of blood flow termed the closure time (CT).
An elevated CT with EPI and collagen can indicate intrinsic defects such as von Willebrand disease, uremia, or circulating platelet inhibitors.
Recently the AABB Industry Standards for Blood Banks and Transfusion Services (5.1.5.1) has allowed use of pathogen reduction technology as an alternative to bacterial screenings in platelets.
Regardless of the initial method of preparation, multiple donations may be combined into one container using a sterile connection device to manufacture a single product with the desired therapeutic dose.
Sometimes a person such as a cancer patient who requires routine transfusions of platelets receives repeated donations from a specific donor to minimize risk.
[73][74] Another photochemical treatment process utilizing amotosalen and UVA light has been developed for the inactivation of viruses, bacteria, parasites, and leukocytes.
[75] In addition, apheresis platelets tend to contain fewer contaminating red blood cells because the collection method is more efficient than "soft spin" centrifugation.
Prior to issuing platelets to the recipient, they may be irradiated to prevent transfusion-associated graft versus host disease or they may be washed to remove the plasma.
Such volume-reduced platelets are normally transfused only to neonatal and pediatric patients when a large volume of plasma could overload the child's small circulatory system.
Local application of these factors in increased concentrations through platelet-rich plasma (PRP) is used as an adjunct in wound healing.