Bonding molecular orbital
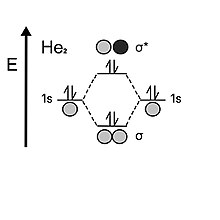
The result of the waves’ constructive interference causes the density of the electrons to be found within the binding region, creating a stable bond between the two species.
Antibonding orbitals are less stable because, with very little to no electron density in the middle, the two nuclei (holding the same charge) repulse each other.
Each electron in the valence 1s shell of hydrogen come together to fill in the stabilizing bonding orbital.
In this case, the electron density of the π orbitals needs to be symmetric along the mirror plane in order to create the bonding interaction.
[6] The delocalized MOs of the carbon atom in the molecule of methane can then be localized to give four sp3 hybrid orbitals.