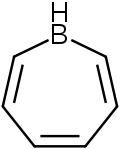
Borepin
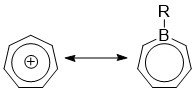
[1][2][3] This leads to an isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acid.
[2][3][4][5][6][7] Simple and complex borepins have been extensively studied more recently due to their high fluorescence and potential applications in technologies like organic light-emitting diodes (OLEDs) and photovoltaic cells.
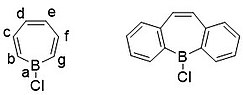
[6][12] The isolated borepin is kinetically stabilized by the bulky phenyl groups bound to all seven positions on the ring, protecting it from reactions with moisture in the air.
Minimal substitution allowed scientists like Ashe to confirm the presence of aromaticity and ring currents within the borepin system.
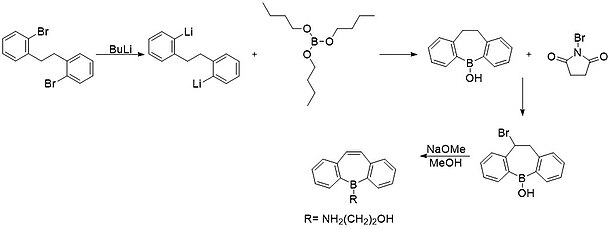
[2][6][13][14] As more modern methods appeared, the tin-boron exchange reaction has become more commonly used as tin can act as a placeholder in the seven-membered ring, reacting with boryl halides quite easily.
[15] While direct functionalization of the boron atom is possible due to its vacant p-orbital, most simple borepins are simply too reactive with air and moisture to be isolated.
Density functional theory (DFT) calculations have shown that the HOMO of borepin lies mostly with the carbon moieties of the seven-membered ring, while the LUMO is centered around the boron atom.
Chemists like Ashe were able to utilize this knowledge in the 1990s to functionalize borepins as a compound, leading to the formation of many Lewis acid-base adducts.
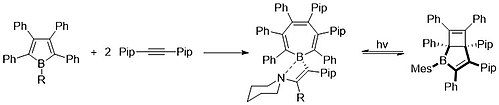
Due to the dative donation of NHCs and CAACs, boron has only two covalent bonds, giving it a formal positive charge.
The electron density shared with the boron center back bonds slightly with the carbon atom, leading to the single-electron radical species.
Some common examples include increasing the number of rings—making boron-doped polycyclic-aromatic hydrocarbons (PAHs), adding additional R groups to the framework such as alkynes and long-chain alkanes, and even introducing electron-rich heteroatoms such as nitrogen or sulfur in order to further stabilize the borepins.
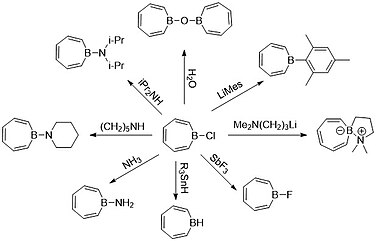
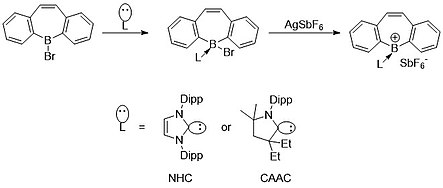
[4][9][11][19][20] Some examples of these compounds can be seen in the image below: The rapid development of borepin stabilization and functionalization since the 2000s has catapulted studies of complex and versatile molecules.
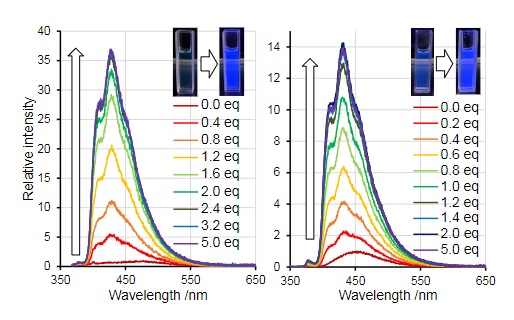
The rationale behind this shift is that the presence of boron in the aromatic system decreases the energy gap between the HOMO and LUMO, resulting in changing absorptions and greater intensity of fluorescence.
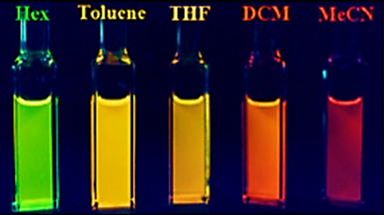
Upon addition of the borepin to hexanes, toluene, tetrahydrofuran (THF), dichloromethane (DCM) and acetonitrile (MeCN), rather drastic changes in color were observed.