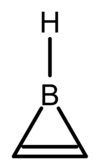
Borirene
First computationally predicted by John Pople and Paul von Rague Schleyer in 1981,[1] the simplest borirene, (CH)2BH, is isoelectronic with the cyclopropenium cation and exhibits Hückel aromaticity.
[2] Borirenes undergo ring-opening reactions with polar reagents[3][4][5] and form Lewis adducts,[6][7][8] due to the significant ring strain in its three-membered structure and the presence of an empty p orbital on the boron atom.
With σ- and π- orbitals close in energy, the HOMO−1 and HOMO (from the σ-framework) can act as a π-donor and σ-donor, respectively, while the LUMO and LUMO+1 serve as both σ-acceptors and π-acceptors.
A detailed computational study on borirenes by Paul von Ragué Schleyer and coworkers have predicted these molecules to undergo facile dimerization into 1,4-diboracyclohexadiene.
[9] Characterized borirenes to date are stabilized predominantly by electron-rich, strong σ- and π-donor substituents that raise the LUMO and lowers the HOMO energy.
Due to their inherent reactivity, sterically demanding groups such as m-terphenyl or mesityl substituents are commonly employed to enhance kinetic stability.
The first identification of a substituted borirene was reported by Van Der Kerk et al., achieved through GC-MS analysis of a one-pot reaction involving MeBBr2, KC8, and di-tert-butylacetylene under reflux conditions.
Similarly, a photochemical approach utilizing tris(triphenylsilyl)borane as a precursor has been reported, wherein a highly reactive silylborylene intermediate reacts with bis(trimethylsilyl)acetylene to form analogous products.
[15] This synthetic pathway is limited by the stability of the starting materials when exposed to strong reducing agents, the inherent instability of the borylene intermediate, and low yields.
As shown in Scheme 2, when an electron-rich metal fragment is bonded to one of the carbon atoms of the borirene, photoinduced rearrangement can occur to yield alkynyl-boranes.
However, they slowly decompose in oxygen, regenerating the original alkyne starting material and yielding trace amounts of a five-membered ring-expansion product, dioxaborole, as detected by GC-MS.[5] Borirenes exhibit promising potential as ligands.
Strong donors, such as isonitriles and N-heterocyclic carbenes, induced ring-expansion and ring-opening reactions, respectively, diverging from the typical Lewis adduct formation observed for borirenes.
More complex transformations were observed with phosphine oxides, aldehydes, and isonitriles, which triggered ring-expansion reactions, leading to the formation of five-membered boron heterocyclic species.