Butanol
The unmodified term butanol usually refers to the straight chain isomer with the alcohol functional group at the terminal carbon, which is also known as 1-butanol.
Butanol exhibits a low order of toxicity in single dose experiments with laboratory animals[2][3] and is considered safe enough for use in cosmetics.
Brief, repeated overexposure with the skin can result in depression of the central nervous system, as with other short-chain alcohols.
In extreme cases this includes suppression of the central nervous system and even death.
[4] A 50% solution of butanol in water has been used since the 20th century to retard the drying of fresh plaster in fresco painting.
2-Methyl-2-butanol is a central nervous system depressant with a similar effect upon ingestion to ethanol.
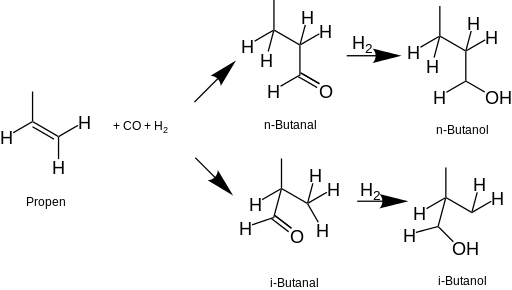
The most common process starts with propene (propylene), which is put through a hydroformylation reaction to form butanal, which is then reduced with hydrogen to 1-butanol and/or 2-butanol.