Carbon–carbon bond
[2] Carbon is one of the few elements that can form long chains of its own atoms, a property called catenation.
This coupled with the strength of the carbon–carbon bond gives rise to an enormous number of molecular forms, many of which are important structural elements of life, so carbon compounds have their own field of study: organic chemistry.
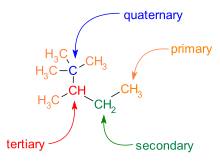
[3] Further, quaternary loci are found in many biologically active small molecules, such as cortisone and morphine.
The directed synthesis of desired three-dimensional structures for tertiary carbons was largely solved during the late 20th century, but the same ability to direct quaternary carbon synthesis did not start to emerge until the first decade of the 21st century.
The values given above represent C-C bond dissociation energies that are commonly encountered; occasionally, outliers may deviate drastically from this range.
Also a consequence of its severe steric congestion, hexakis(3,5-di-tert-butylphenyl)ethane has a greatly elongated central bond with a length of 167 pm.