Organocatalysis
[3][4][5][6][7][8] Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
Organocatalysts which display secondary amine functionality can be described as performing either enamine catalysis (by forming catalytic quantities of an active enamine nucleophile) or iminium catalysis (by forming catalytic quantities of an activated iminium electrophile).
[10] Organic chemists David MacMillan and Benjamin List were both awarded the 2021 Nobel Prize in chemistry for their work on asymmetric organocatalysis.
The starting material is an achiral triketone and it requires just 3% of proline to obtain the reaction product, a ketol in 93% enantiomeric excess.
[18] A mini-review digest article focuses on selected recent examples of total synthesis of natural and pharmaceutical products using organocatalytic reactions.
A breakthrough in the field of organocatalysis came in 1997 when Yian Shi reported the first general, highly enantioselective organocatalytic reaction with the catalytic asymmetric epoxidation of trans- and trisubstituted olefins with chiral dioxiranes.
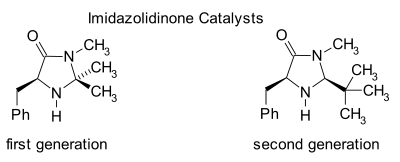
This catalyst works by forming an iminium ion with carbonyl groups of α,β-unsaturated aldehydes (enals) and enones in a rapid chemical equilibrium.