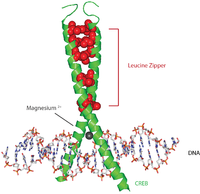
CREB
It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the genes.
[4] CREB is closely related in structure and function to CREM (cAMP response element modulator) and ATF-1 (activating transcription factor-1) proteins.
[7] When activated, CREB protein recruits other transcriptional coactivators to bind to CRE promoter 5’ upstream region.
However, the majority of these sites remain unbound due to cytosine methylation, which physically obstructs protein binding.
Flies genetically engineered to overexpress the inactive form of CREB lose their ability to retain long-term memory.
[17] The function of CREB can be modulated via a signalling pathway resulting from the binding of serotonin and noradrenaline to post-synaptic G-protein coupled receptors.
Light excites melanopsin-containing photosensitive retinal ganglion cells which signal to the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract (RHT).
[19] The phosphorylated CREB recognizes the cAMP Response Element and serves as a transcription factor for Per1 and Per2, two genes that regulate the mammalian circadian clock.
In vivo, phase shift-inducing light pulses during the subjective night correlated with CREB phosphorylation in the SCN.
[20] Experiments by Gunther Schutz in 2002 demonstrated that mutant mice lacking the Ser142 phosphorylation site failed to induce the clock regulatory gene mPer1 in response to a light pulse.